You may well be allowed to publish an Ethicon surgery staples lawsuit on the flawed service or product. In October 2019, the U.S. Foods and Medicine Administration (Food and medicine management) started a Class 1 recall—its most severe form of recall—of Ethicon operative staplers. In accordance with the federal business, the merchandise falters to properly type staples which may boost the risk of health-related difficulties.
There have reportedly been quite a few traumas a result of Ethicon surgery stapler difficulties along with one dying. Get in touch with an Ethicon Keep in mind Lawyer to view in case you complement the standards for the Ethicon lawsuit staples.
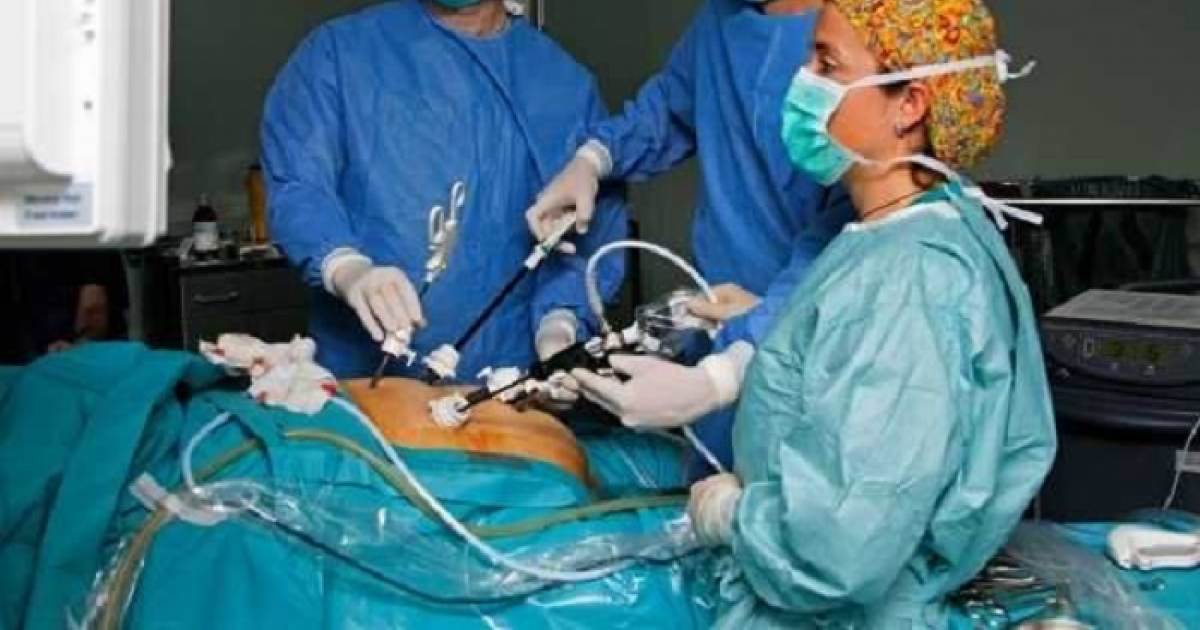
Ethicon Operative Stapler Remember
There were quite a few recalls of operative staplers over time, affirming precisely how great-chance they could be to utilise. In May 2013, Ethicon, a subdivision of drug huge Johnson & Johnson, granted a category-II keep in mind for surgical procedure staples spread under the label Echelon. Echelon staples were actually utilized in many different types of surgical procedure, such as abdomen stapling surgical treatment. The bear in mind implies that Echelon staples utilized in tummy stapling finished up becoming noted to misfire and bust, jeopardizing severe issues.
On Oct. 30, 2019, the Federal medication administration released the course 1 remember of Ethicon’s Echelon Flex Endopath staplers. The Government drug management keep in mind, commenced with all the Johnson & Johnson subsidiary earlier in October, contains numerous goods, such as the seeking:
ECHELON Flex 60 Endopath Stapler, Articulating Endoscopic Linear Cutter (EC60A)
ECHELON Flex 60 Run Plus Little Articulating Endoscopic Linear Cutter (PCEE60A)
ECHELON Flex 60 Powered Plus Articulating Endoscopic Linear Cutter, 44cm Shaft Size (PLEE60A)
ECHELON Flex 60 Manage Plus Articulating Endoscopic Linear Cutter, 34cm Shaft Period (PSEE60A)
These individual-buyer staplers were actually created to use on inside tissue throughout minimally intrusive gynecologic, urologic, thoracic, pediatric, and regular operative surgical procedures.
As outlined by the Federal drug administration, these items could have a factor that has run out of specification—leading to malformed staples. Malformed staples may lead to serious surgery difficulties.Two patients was reportedly wounded from the standard gadgets when a misfire bring about their rectums simply becoming minimize. By Oct. 3, Ethicon has reportedly received seven reviews of significant incidents and something document of dying.



